PLOS Medicine: Preventing microalbuminuria with benazepril, valsartan, and benazepril–valsartan combination therapy in diabetic patients with high-normal albuminuria: A prospective, randomized, open-label, blinded endpoint (PROBE) study

Study protocol for a phase III multicentre, randomised, open-label, blinded-end point trial to evaluate the efficacy and safety of immunoglobulin plus cyclosporin A in patients with severe Kawasaki disease (KAICA Trial)

Methodology of a large prospective, randomised, open, blinded endpoint streamlined safety study of celecoxib versus traditional non-steroidal anti-inflammatory drugs in patients with osteoarthritis or rheumatoid arthritis: protocol of the standard care ...
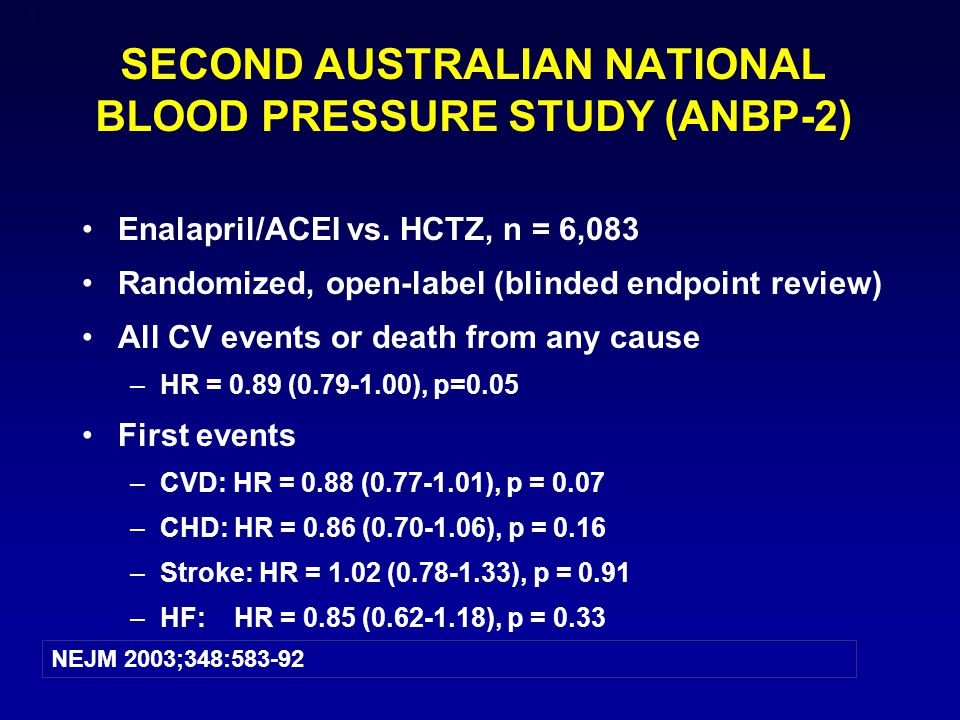
1 SECOND AUSTRALIAN NATIONAL BLOOD PRESSURE STUDY (ANBP-2) Enalapril/ACEI vs. HCTZ, n = 6,083 Randomized, open-label (blinded endpoint review) All CV events. - ppt download
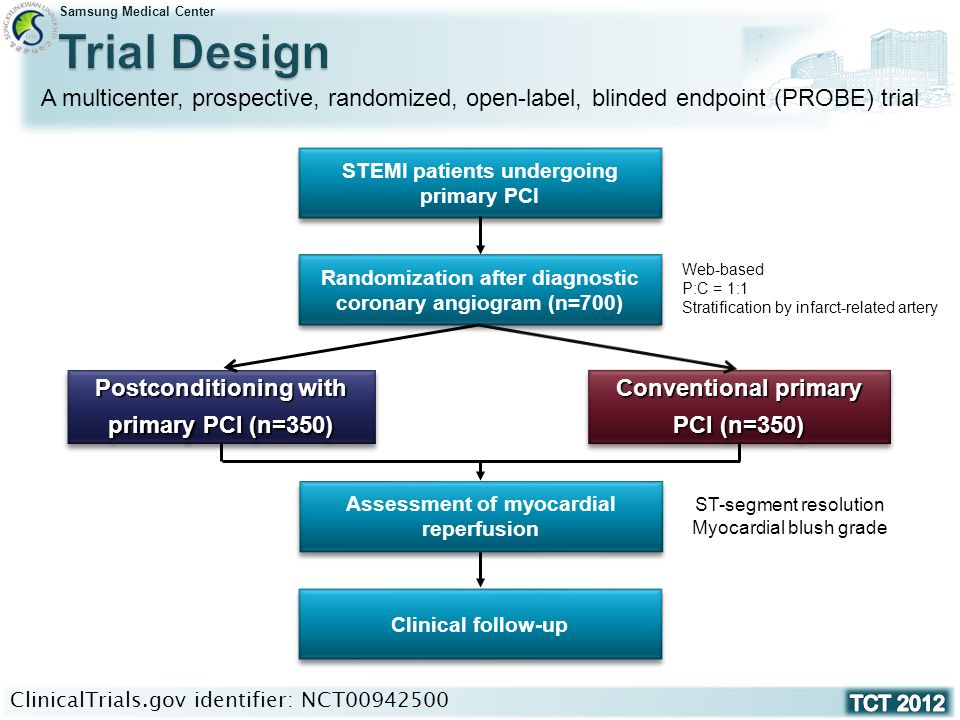
Effect of Postconditioning on Myocardial Reperfusion during Primary Percutaneous Coronary Intervention Joo-Yong Hahn / Hyeon-Cheol Gwon On behalf of the. - ppt video online download
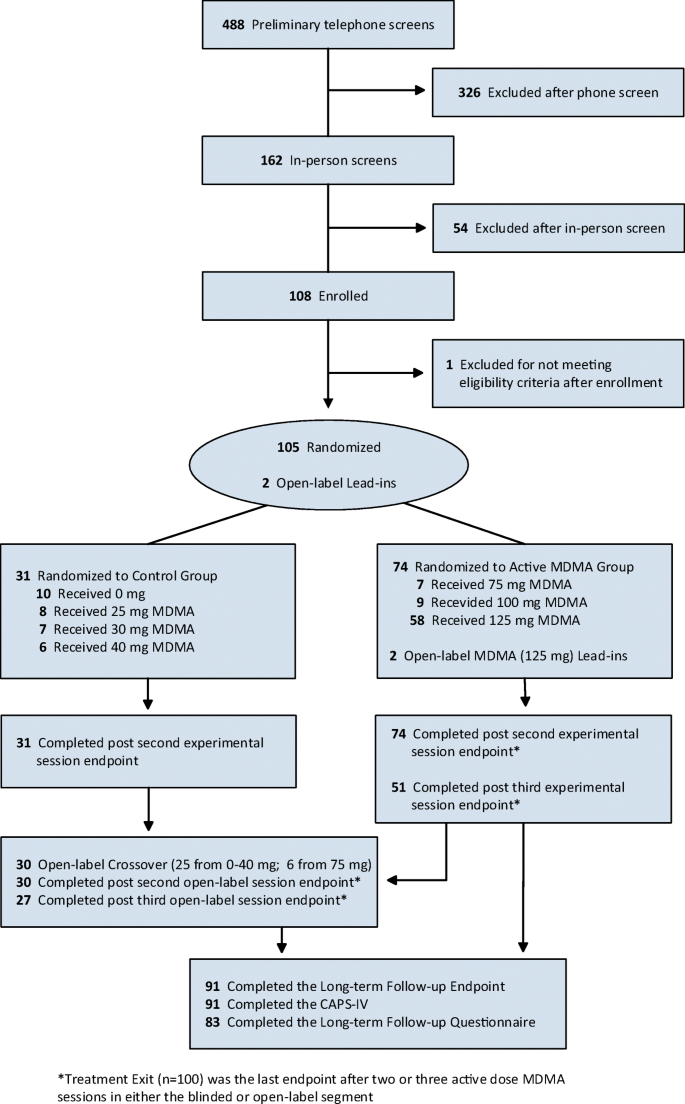
Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials | SpringerLink

Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial - The Lancet

A Prospective, Randomized, Open-Label, Blinded, Endpoint Study Exploring Platelet Response to Half-Dose Prasugrel and Ticagrelor in Patients with the Acute Coronary Syndrome: HOPE-TAILOR Study | Semantic Scholar

PDF) GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded end point (PROBE) trial
![PDF] GI-REASONS: A Novel 6-Month, Prospective, Randomized, Open-Label, Blinded Endpoint (PROBE) Trial | Semantic Scholar PDF] GI-REASONS: A Novel 6-Month, Prospective, Randomized, Open-Label, Blinded Endpoint (PROBE) Trial | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a0653b7b38e796366a33a888639ab58e6f15b68e/4-Figure1-1.png)
PDF] GI-REASONS: A Novel 6-Month, Prospective, Randomized, Open-Label, Blinded Endpoint (PROBE) Trial | Semantic Scholar

Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study | BMJ Open

PPT - Blinding or Masking of Treatments and Other Aspects of the Trial PowerPoint Presentation - ID:2947805

Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial - The Lancet

Bioavailability of everolimus administered as a single 5 mg tablet versus five 1 mg tablets: a randomized, open-label, two-way crossover study of healthy volunteers

A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome - ScienceDirect

A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome - ScienceDirect
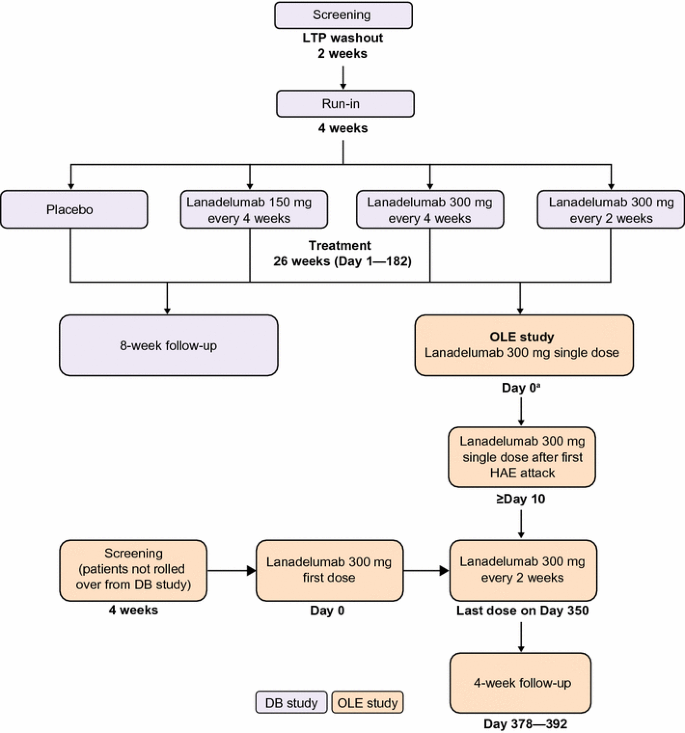
An open-label study to evaluate the long-term safety and efficacy of lanadelumab for prevention of attacks in hereditary angioedema: design of the HELP study extension | Clinical and Translational Allergy | Full

Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: A prospective, multicentre, open‐label, blinded‐endpoint, randomized ...

Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial - The Lancet Neurology