A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia - Atherosclerosis

Pharmacokinetic Interaction Between Rosuvastatin and Metformin in Healthy Korean Male Volunteers: A Randomized, Open-label, 3-period, Crossover, Multiple-dose Study - Clinical Therapeutics

Pharmacokinetics of rosuvastatin in healthy Chinese volunteers living in China: a randomized, open-label, ascending single- and multiple-dose study. | Semantic Scholar

Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial - eClinicalMedicine

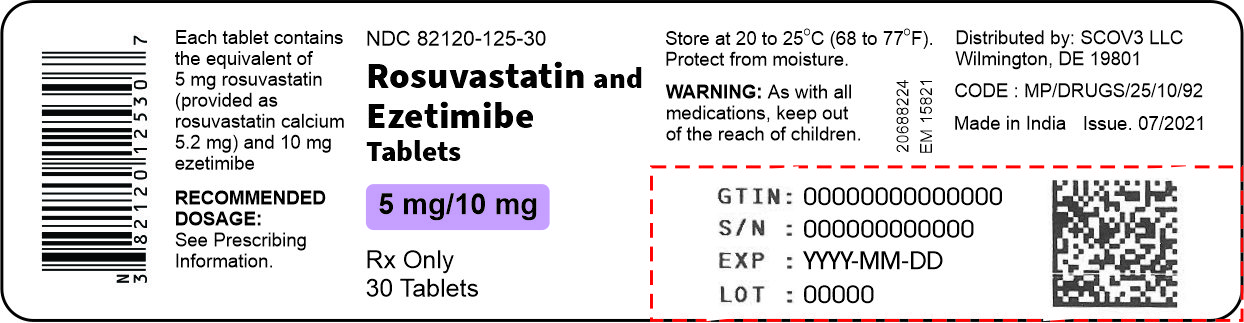
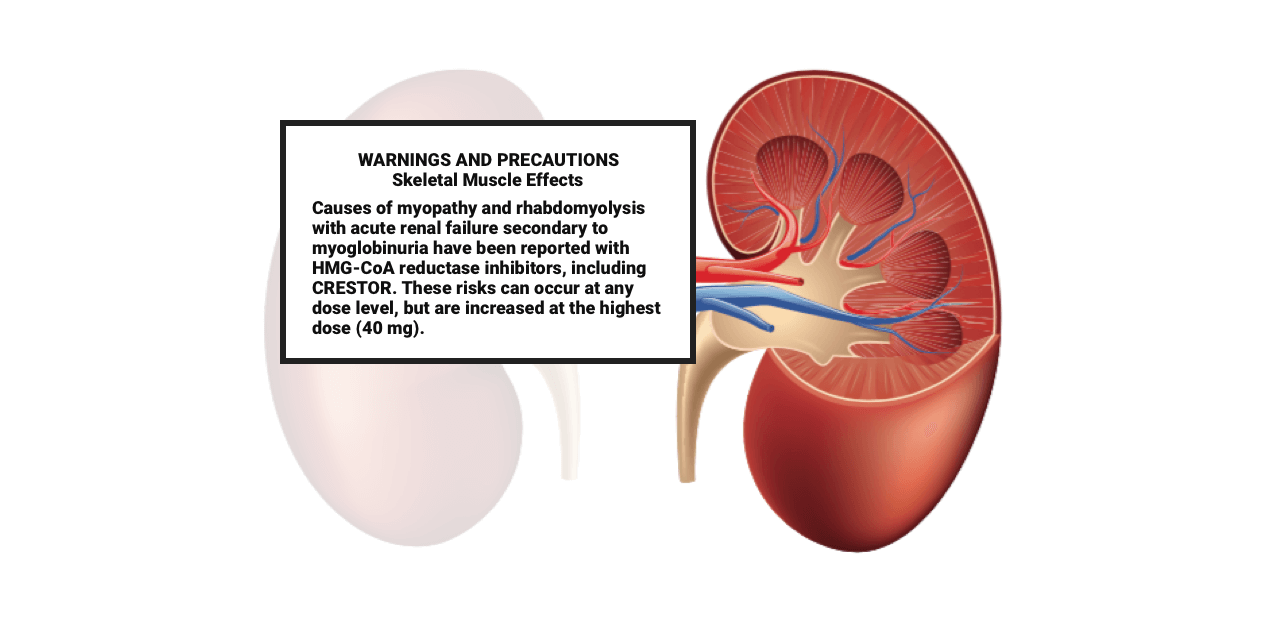
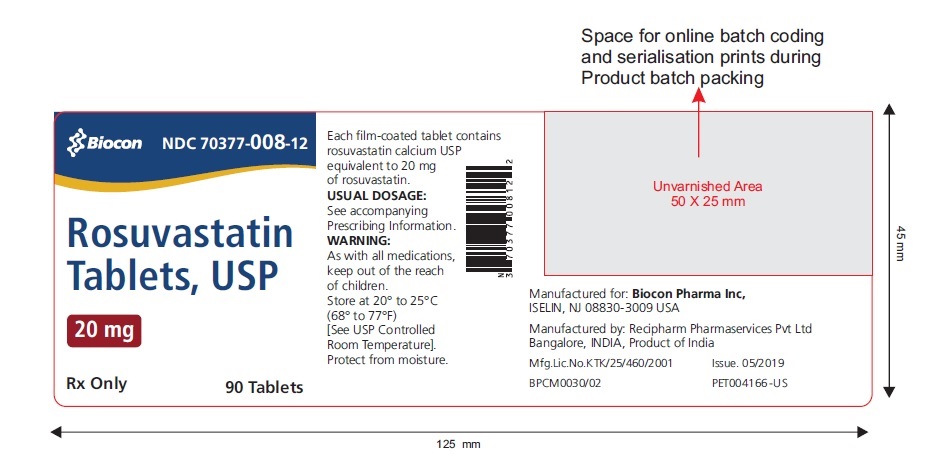
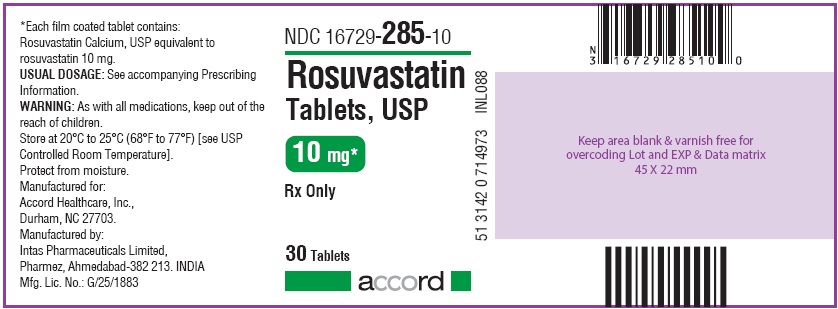
These highlights do not include all the information needed to use ROSUVASTATIN TABLETS safely and effectively. See full prescribing information for ROSUVASTATIN TABLETS. ROSUVASTATIN tablets, for oral use Initial U.S. Approval: 2003

Pharmacokinetic interaction between rosuvastatin and olmesartan: a randomized, open-label, 3-period, multiple-dose crossover study in healthy Korean male subjects. | Semantic Scholar